ETIOLOGY OF ISCHEMIC STROKE
Paradoxical embolism and stroke
Updated on 22/09/2024, published on 20/06/2024
The diagnosis of a paradoxical embolus can be confidently made only when the following four criteria are met:
- venous thrombus (or other material) as a source of emboli
- venous thrombi typically form in the deep veins of the legs or pelvis due to factors such as prolonged immobility, hypercoagulable states, or venous injury
- venous thrombosis must be reasonably distinguished as being a cause rather than a consequence of stroke (especially if detected > 3 days from stroke onset)
- a shunt (PFO, atrial septal defect, PAVM) that provides a pathway for the embolus to bypass the pulmonary filter and enter the arterial circulation
- a pressure gradient promoting right to left shunt
- elevated right atrial pressures, commonly seen during Valsalva maneuvers (urination, defecation), increase the likelihood of shunt opening
- a thrombus lodged in the arterial circulation
- the embolus can travel to various organs, causing ischemic events such as strokes, myocardial, mesenteric, or limb ischemia
- proving that a stroke is caused by a paradoxical embolism is often difficult
- rarely are all four of the above criteria diagnosed with high certainty
- diagnosis is typically made per exclusionem
- however, if recent deep vein thrombosis is found along with a significant PFO or a thrombus in the PFO tunnel in a stroke patient, paradoxical embolization is very likely
- due to diagnostic uncertainty, some strokes caused by paradoxical embolism may be misclassified as cryptogenic stroke
Classification of shunts
| Cardiac shunts | Extracardiac shunts |
|
PFO atrial septal defects (ASD) ventricular septal defects (VSD) truncus arteriosus |
pulmonary A-V malformation or fistula persistent left superior vena cava (PLSVC) |
Congenital cardiac defects
Patent Foramen Ovale (PFO)
- anatomic closure of the foramen ovale normally follows functional closure after birth. However, autopsy studies have found that a patent interatrial communication remains in a significant proportion of healthy subjects (up to 25-30% have PFO with a mean diameter of 5 mm )
- echocardiographic methods underestimate the prevalence and size of PFOs compared to autopsy findings (prevalence ~ 10-22%)
- PFO is more prevalent in patients with cryptogenic stroke compared to patients with stroke of determined origin or healthy individuals
- the coexistence of other cardiac findings strengthens the association between PFO and paradoxical embolism:
- concomitant atrial septal aneurysm (ASA)
- ASA may promote local thrombosis at the site of dilatation
- ASA may partially reflect a larger PFO size
- flapping motion of the ASA may direct small thrombi coming from the inferior vena cava (IVC) into a PFO
- persistent prominent eustachian valve and right atrial filamentous strands
- concomitant atrial septal aneurysm (ASA)
- rarely a thrombus can be found in the PFO tunnel (either traveling from the venous system or formed locally)
- PFO size does not appear to be related to the risk of stroke recurrence
- there is no specific brain imaging pattern associated with paradoxical embolization
Atrial Septal Defect (ASD)
- a congenital defect in the atrial septum, allowing direct communication between the right and left atria, leading to a mix of oxygenated and deoxygenated blood
- over time, this can cause enlargement of the right atrium and right ventricle, pulmonary hypertension, and potentially right heart failure
- in some cases, the shunt can reverse (right-to-left), causing cyanosis (due to the mixing of deoxygenated blood in the systemic circulation) or paradoxical embolization
- types of ASD based on their location within the atrial septum:
- ostium secundum ASD – the most common type, located in the middle of the atrial septum (75% of cases)
- ostium primum ASD – located in the lower part of the atrial septum, often associated with mitral valve defect and ventricular septal defect
- sinus venosus ASD – located near the superior vena cava or inferior vena cava junction with the right atrium
- coronary sinus ASD – a rare type where the defect is near the coronary sinus
- ASD is usually diagnosed with an echocardiogram, which can show the size and location of the defect and its effect on the heart
- management of ASD depends on the size of the defect and the presence of symptoms
- observation (small, asymptomatic ASDs)
- medical management (arrhythmias or heart failure)
- transcatheter closure or surgical repair
- moderate to large ASDs
- in symptomatic patients (shortness of breath, easy fatigability, or palpitations) (ACC/AHA 2008 IIa/C)
- in patients at risk of developing pulmonary hypertension (high blood pressure in the lungs)
- ASD (irrespective of size, except for very small ASD in older patients) + history of paradoxical embolism
- moderate to large ASDs
Ventricular Septal Defect (VSD)
- a congenital heart defect characterized by an abnormal opening in the ventricular septum; this defect allows blood to flow between the ventricles, leading to a mix of oxygenated and deoxygenated blood and altering normal hemodynamics
- this leads to increased pulmonary blood flow, elevated right ventricular pressure, and volume overload of the left atrium and left ventricle
- over time, this can cause pulmonary hypertension, left ventricular hypertrophy, and congestive heart failure
- in severe cases, the shunt may reverse (Eisenmenger syndrome), leading to cyanosis and right-to-left shunting
- VSDs can occur as isolated defects or in conjunction with other congenital heart anomalies, such as tetralogy of Fallot or Down syndrome
- VSDs are classified based on their location within the ventricular septum:
- perimembranous VSD – the most common type, located in the membranous portion of the septum, near the tricuspid and aortic valves
- muscular VSD – located in the muscular part of the septum and can occur anywhere within the septum; these defects can be multiple
- inlet VSD – located near the tricuspid and mitral valves, associated with the atrioventricular canal
- outlet VSD – located near the pulmonary and aortic valves, also known as supracristal or subpulmonary VSD
- the management of VSD depends on the size of the defect, the presence of symptoms, and the hemodynamic impact ⇒ medical management, surgical repair, percutaneous closure
Pulmonary A-V shunts
- pulmonary arteriovenous shunts (PAVMs) are abnormal connections between pulmonary arteries and veins, bypassing the normal capillary system ⇒ hypoxemia, paradoxical embolism, increased cardiac output
- etiology
- congenital PAVMs (associated with hereditary hemorrhagic telangiectasia (HHT), also known as Osler-Weber-Rendu syndrome. HHT is an autosomal dominant genetic disorder characterized by abnormal blood vessel formation)
- acquired PAVMs – less common, can occur due to conditions such as trauma, metastatic cancer, or as a complication of certain medical procedures like lung transplantation or thoracic surgery
- this results in a direct passage of blood from the arterial to the venous system without oxygenation in the lungs, leading to various clinical complications
- symptoms vary depending on the size and number of shunts:
- asymptomatic – small PAVMs may be asymptomatic and discovered incidentally during imaging for other reasons
- hypoxemia and increased cardiac output – dyspnea, cyanosis, fatigue, and exercise intolerance
- complications due to paradoxical emboli (strokes, brain abscesses, systemic embolic events, etc.)
- hemoptysis – rare
- diagnostic evaluation
- chest X-ray – nodular or mass-like lesions
- contrast echocardiography or TCCD bubble test – used to detect right-to-left shunts
- HRCT + pulmonary CTA – considered the gold standard for diagnosing PAVMs, allowing direct visualization of the shunt
- management: embolization, resection (typically where embolization is not feasible or unsuccessful)
Rare anomalies
Persistent left superior vena cava (PLSVC)
- a rare vascular anomaly that begins at the junction of the left subclavian and internal jugular veins and passes through the left side of the mediastinum adjacent to the aortic arc
- it mostly drains into the right atrium via the coronary sinus (CS) but may also terminate in the left atrium
- PLSVC is mostly asymptomatic and detected incidentally; however, it can be discovered as a part of complex cardiac pathologies and may lead to significant issues such as arrhythmias or paradoxical embolization (particularly if it drains into the left atrium or if there is a concurrent PFO)
Truncus arteriosus
- a rare congenital heart defect characterized by a single arterial trunk arising from the heart, supplying blood to both the systemic and pulmonary circulations.
- it is associated with other anomalies
- varying origins of the pulmonary arteries from the trunk
- atrial or ventricular septal defect (VSD)
- patent ductus arteriosus
- heart surgery is required within the first weeks of life
- rarely, some individuals (usually with type 1) can survive without surgery; they typically develop heart failure and Eisenmenger syndrome (Abid, 2015)
- type I (50-70%):
- a short main pulmonary trunk arises from the truncus arteriosus; this trunk then branches into right and left pulmonary arteries
- type II (20-30%):
- no main pulmonary trunk
- right and left pulmonary arteries arise separately but close together from the posterior aspect of the truncus
- type III:
- no main pulmonary trunk
- right and left pulmonary arteries arise separately from widely separated points on the lateral aspects of the truncus
- type IV:
- no true pulmonary arteries arise from the truncus
- pulmonary blood supply comes entirely from major aortopulmonary collateral arteries
- now often classified separately as pulmonary atresia with ventricular septal defect (PA-VSD)
Venous thrombus sources
- detection of a venous thrombosis influences the likelihood of diagnosing a cryptogenic stroke due to paradoxical embolism, but the venous evaluation is often incomplete
- some methods may lack the sensitivity to consistently detect all relevant venous thrombi
- clinical diagnosis of DVT is unreliable
- routinely used laboratory screening tests, such as D-dimer, have high sensitivity but very low specificity
- contrast venography and ultrasonography are sensitive for detecting DVT in the thigh but not in the pelvis
- the sensitivity of contrast venography for pelvic vein pathology can be improved by selectively cannulating these veins, though this is infrequent due to the procedure’s invasiveness
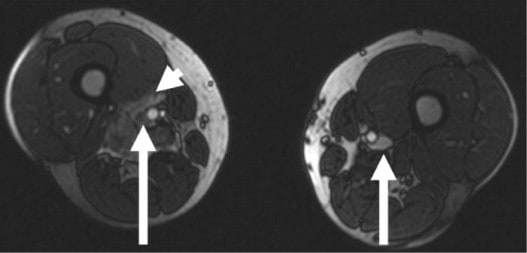
- it is essential to evaluate calf veins in stroke patients (in general medical practice, little emphasis is placed on the treatment of calf vein DVT without extension to popliteal veins)
- in some institutions, lower extremity venous duplex examinations do not include the study of calf vein
- embolization can occur even without propagation to proximal segments
- emboli from calf vein thrombi tend to be small and asymptomatic when reaching the lung but may become clinically significant in the cerebral circulation
- another issue in the context of paradoxical stroke is the evaluation of the pelvic veins (use MR venography)
- the most commonly involved pelvic veins are the external iliac vein and the common iliac vein
- thrombosis in superficial lower extremity veins is also associated with pulmonary embolism in a surprising proportion of cases
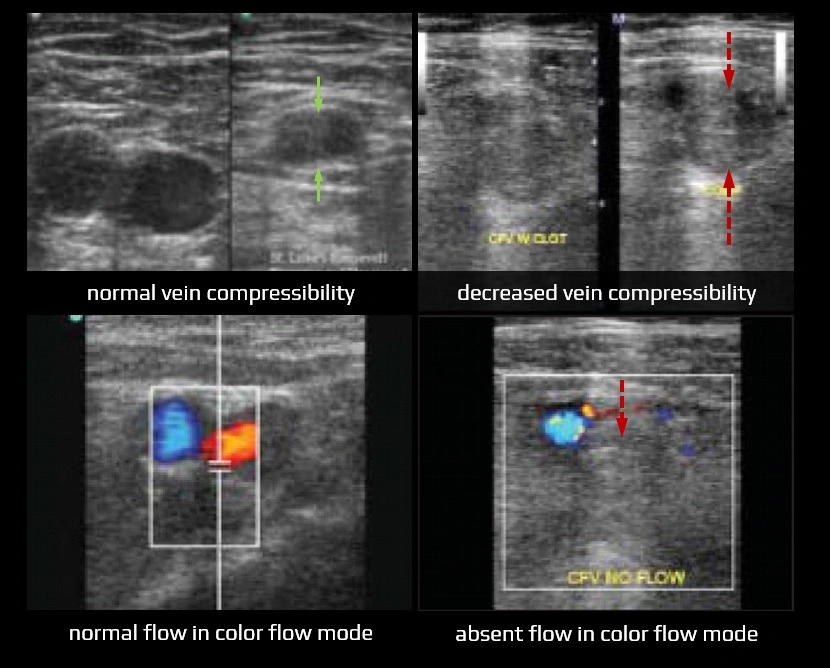
- diagnostic ultrasound criteria for DVT
- primary: non-compressibility of the vein
- secondary: echogenic thrombus within the vein lumen, venous distention, complete absence of spectral or color Doppler signal within the vein lumen, and loss of response to Valsalva or augmentation
- in acute thrombosis, the vein is distended by hypoechoic thrombus and shows partial or no compressibility without collaterals
- improved diagnostic methods of the venous system include:
- MR/CT venography offers the advantage of evaluating the pelvic veins and inferior vena cava (IVC), which are difficult to assess with ultrasound
- MR direct thrombus imaging (DTI) (Westerbeek, 2008)
- venous enhanced subtracted peak arterial MRV (Frazer, 2003)
- an important concern is a substantial increase in DVT prevalence that is found beginning approximately day 4 poststroke (such thrombosis is usually a consequence rather than a cause of the stroke)
Therapeutical considerations
- acute stroke therapy should follow established protocols
- prevention of stroke recurrence depends on the certainty of paradoxical embolism involvement
- when paradoxical embolism is confirmed with high confidence (e.g., presence of PFO with associated thrombus or concurrent DVT), PFO closure is considered (unless there is a compelling reason for long-term anticoagulation)
- however, in most cases, the evidence for a paradoxical embolism is circumstantial and/or incomplete, and uncertainties exist at several levels
Management strategies include:
- conservative approach (small shunt on TCCD bubble test, questionable causal connection of the shunt with the current stroke)
- antiplatelet therapy
- anticoagulation (cases of VTE, hypercoagulable state)
- intervention (massive shunt with a high probability of paradoxical embolization)
- percutaneous closure (PFO, ASD)
- embolization (PAVM)
- surgical intervention – in cases where percutaneous closure is not feasible
Stroke following closure treatment
- recurrent stroke may occur despite the closure of the source of paradoxical embolism (PFO, ASAD, etc.)
- causes vary depending on the timing of the event
Early stroke (< 30 days)
- thrombus formation on the occluder with subsequent peripheral embolism
- device malposition
- periprocedural atrial arrhythmias
- device may induce atrial fibrillation, increasing the risk of atrial thrombus formation
- periprocedural air embolism
Delayed stroke (> 30 days)
- delayed thrombus formation on the device
- residual shunts or incomplete closure with repeated paradoxical embolism
- the residual hole may allow venous clots to pass into the arterial circulation
- regular imaging is used to assess device position and to detect clots or residual shunts
- persistent or paroxysmal atrial fibrillation
- stroke from another cause (which may have been missed prior to occlusion)